An international research team has demonstrated how the simultaneous measurement of dozens of types of fats in the blood can predict the risk of developing type 2 diabetes (T2D) and cardiovascular disease (CVD) years in the future. The investigators at Lipotype, Lund University, and Twincore Centre for Experimental and Clinical Infection Research suggest that such early prediction through lipidomic profiling may provide the basis for recommending diet and lifestyle interventions before disease develops.
Lipotype research lead Chris Lauber, PhD, stated, “The lipidomic risk, which is derived from only one single mass-spectrometric measurement that is cheap and fast, could extend traditional risk assessment based on clinical assay … Strengthening disease prevention is a global joint effort with many facets. We show how lipidomics can expand our toolkit for early detection of individuals at high risk of developing diabetes and cardiovascular diseases.”
Lauber and colleagues reported on their study in PLOS Biology, in a paper titled, “Lipidomic risk scores are independent of polygenic risk scores and can predict incidence of diabetes and cardiovascular disease in a large population cohort,” in which they concluded, “For both diseases, we observe a subgroup of individuals with extraordinarily high risk scores and demonstrate that these subgroups are associated with large-scale alternations of the lipidome that may be prognostic for future disease incidence.”
Cardiovascular diseases and diabetes mellitus are among the 10 leading causes of death globally, the authors noted, citing WHO reports. The risk of developing CVD and type 2 diabetes is associated with diet and other lifestyle-related behaviors and so the ability to identify, early, individuals at high disease risk could be crucial for lowering the disease burden, through the suggestion of potential ways of reducing that risk, such as changes to the diet.
Current assessment of risk for T2D and CVD relies largely on patient history and current risk behaviors, and the levels and ratio of two major blood lipids, high- and low-density cholesterol. But the blood contains over one hundred other types of lipids, which are thought to reflect at least in part aspects of metabolism and homeostasis throughout the body.
“Risk prediction based on machine learning models has been shown to benefit from the inclusion of omics-based measurements in addition to the classical risk factors such as cholesterol and glucose levels in the blood,” the investigators pointed out. “In particular, assessing variations in the genome, proteome, and metabolome including the lipidome may offer opportunities to identify pathophysiological processes and pathway, which may differ between patients or patient subgroups.”
Interestingly, they continued, recent research has demonstrated that lipids are sensitive metabolic indicators of change in health and disease. “Most encouraging is that the blood plasma lipidome seems to reflect the metabolic status in the body and plasma offers an easily accessible resource for lipidomic analysis.”
For their newly reported study, which aimed to assess whether a more comprehensive measure of blood lipids could increase the accuracy of risk prediction, the authors drew on data and blood samples from a longitudinal health study of over 4,000 healthy, middle-aged Swedish residents, first assessed from 1991 to 1994, and followed until 2015. “Here we integrated genetics, lipidomics, and standard clinical diagnostics to assess future T2D and CVD risk for 4,067 participants from a large prospective population-based cohort, the Malmö Diet and Cancer-Cardiovascular Cohort,” they noted.
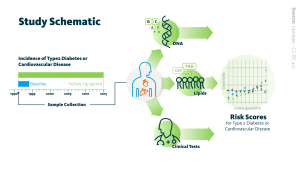
The team established a shotgun mass spectrometric platform for lipidomics analysis, which they claim performs at high-throughput, reproducibly, and quantitatively with high precision. Using baseline blood samples, the concentrations of 184 lipids were then assessed using the quantitative mass spectrometry approach. During the follow-up period, 13.8% of participants developed T2D, and 22% developed CVD.
To develop the lipid-based risk profile, the authors performed repeated training/test rounds on the data, using a randomly chosen two-thirds of lipid data to create a risk model, and then seeing if the model accurately predicted risk in the remaining third.
Once the model was developed, individuals were clustered into one of six subgroups based on their lipidomics profile. “By training Ridge regression-based machine learning models on the measurements obtained at baseline when the individuals were healthy, we computed several risk scores for T2D and CVD incidence during up to 23 years of follow-up,” the team commented. “We used these scores to stratify the participants into risk groups ….”
The findings showed that, compared to the group averages, the risk for T2D in the highest-risk group was 37%, an increase in risk of 168%. The risk for CVD in the highest-risk group was 40.5%, an increase in risk of 84%. The increased risk for either disease was independent of known genetic risk factors, and independent of the number of years until disease onset. “Notably, lipidomic risk correlated only marginally with polygenic risk, indicating that the lipidome and genetic variants may constitute largely independent risk factors for T2D and CVD,” the investigators added.
Conversely, there were significant reductions in risk, compared to the averages, in the lowest-risk groups, and these were calculated as “… a 77% and 53% decrease of the incidence rate in the lowest risk group for T2D and CVD, respectively, compared to the average case rates of 13.8% and 22.0%.”
Risk stratification was then further improved by adding standard clinical variables to the model, and this resulted in a case rate of 51.0% and 53.3% in the highest risk group for T2D and CVD, respectively, the investigators pointed out. Interestingly, participants in the highest risk group showed significantly altered lipidome compositions affecting 167 lipid species for T2D and 157 for CVD.
The authors concluded, “Our results demonstrated that a subset of individuals at high risk for developing T2D or CVD can be identified years before disease incidence.” The lipidomic risk, which is derived from only one single spectrometric measurement that is cheap and fast, is informative and could extend traditional risk assessment based on clinical assays.”
There are several potentially important implications of these findings. On an individual level, it may be possible to define risk decades before disease onset, possibly in time to take steps to avert disease. Lipidomics, either in combination with genetics and patient history or independent of them, may provide new insights into when and why disease begins. In addition, by identifying those lipids that contribute most to risk, it may be possible to identify new drug candidates.
Individual lipids in the blood may be the consequences of or contribute to a wide variety of metabolic processes, which may be individually significant as markers of those processes. If that is true, Lauber said, “the lipidome may provide insights much beyond diabetes and cardiovascular disease risk.”

![BI will develop firm’s dual glucagon/GLP-1 agonist and develop new dual agonists.[evgenyb - Fotolia.com] BI will develop firm’s dual glucagon/GLP-1 agonist and develop new dual agonists.[evgenyb - Fotolia.com]](https://www.genengnews.com/wp-content/uploads/2018/08/June16_2011_2346720_DiabetesFingerPrickCloseUp_BIZealandCollab1451667021.jpg)

